Bacterial Biofilms Can Take on Some Animal-Like Characteristics
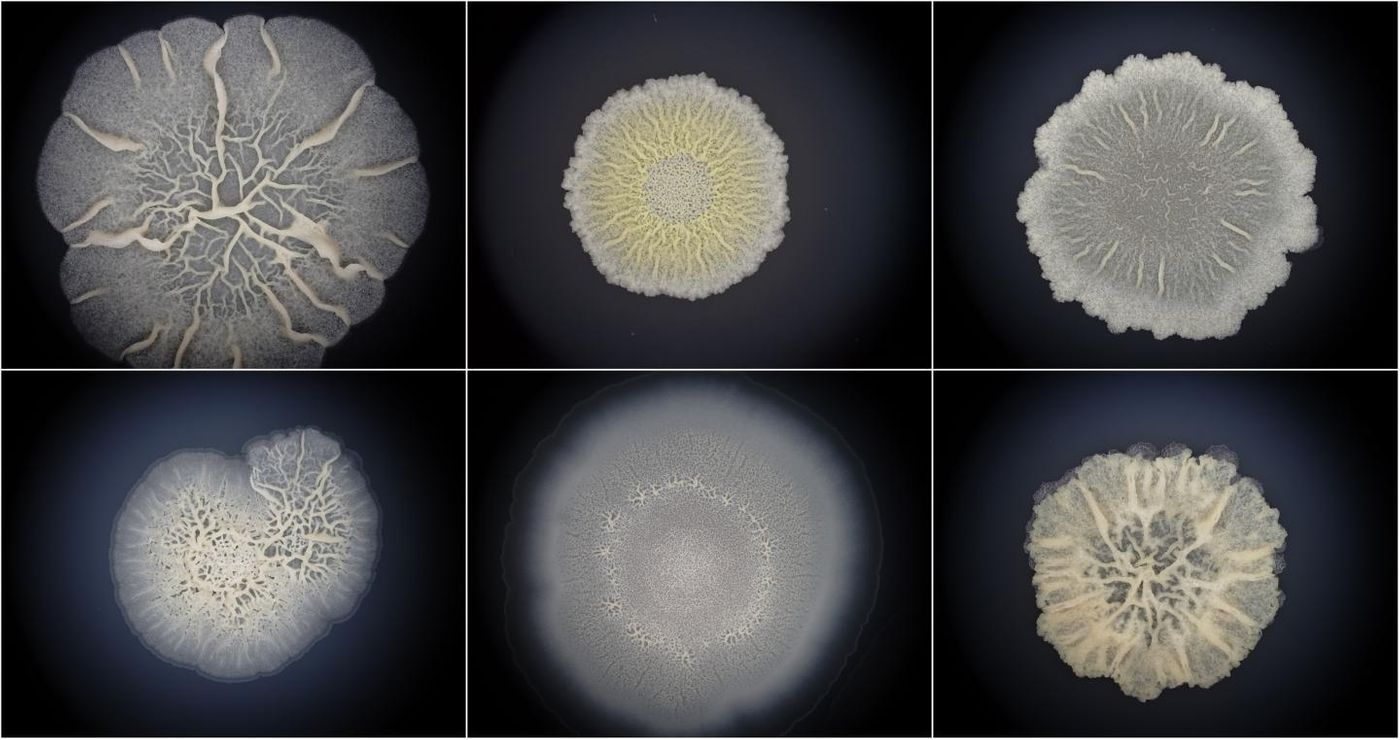
Bacteria are everywhere, even inside of our bodies, and they are thought to date back to the early days of life on Earth. These single-celled organisms are classified differently than animals' eukaryotic cells on the tree of life. New work has suggested that bacteria have more in common with animals than we knew, however. Reporting in Molecular Biology and Evolution, researchers have examined the nature of bacterial communities called biofilms and found that their development parallels that of multicellular organisms.
"Surprisingly, we found that the development of bacterial biofilms is comparable to animal embryogenesis. This means that bacteria are true multicellular organisms just like we are. Considering that the oldest known fossils are bacterial biofilms, it is quite likely that the first life was also multicellular, and not a single-celled creature as considered so far," suggested the study leader Tomislav Domazet-Lošo from the Rudjer Boskovic Institute and the Catholic University of Croatia in Zagreb.
More evidence will be needed to support this hypothesis, since biofilms do not form organ systems like many familiar animals. Biofilms may turn out to have many parallels with the simplest animals that have no organs, like Trichoplax adhaerans, which behaves like it has a nervous system though it does not.
While many bacteria live in symbiosis with other organisms, the vast majority live on their own in microbial communities. These biofilms are known to take on characteristics that individual microbes do not have. For example, biofilms are much tougher and sturdier than individual microorganisms, making biofilms far more difficult to eliminate. We know that biofilms can grow on implanted medical devices, where they can pose serious health risks. But many other microbes can live in the human body, and researchers don’t know much about the biofilms they might form.
"Evolutionary methods to study collective behavior of cells in animal development were at hand, but no one tried to transfer this technology from animal embryos to bacterial biofilms. Perhaps people were uncomfortable to challenge the special status of animal multicellularity, the idea that is culturally hardwired," noted Domazet-Lošo.
Previous work of Domazet-Lošo and colleagues has investigated ontogeny and phylogeny using a computational approach called genomic phylostratigraphy. Their research suggested that the early development of individuals mirrors the development of their ancestors. The genomic phylostratigraphy tool enables the team to apply an evolutionary date to genes and proteins on a large scale.
In this work, they teamed up with researchers from the University of Zagreb, Chalmers University, and the Technical University of Denmark to apply the method on bacteria. "We generated the first phylostratigraphic maps of bacteria and this allowed us to link bacterial phenotypes in biofilms to evolutionary information," said Domazet-Lošo.
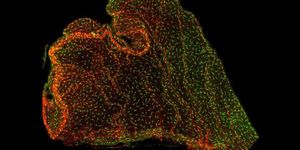
The team obtained data from Bacillus subtilis biofilms and quantified the levels of all B. subtilis genes while biofilms formed.
"Surprisingly, we found that evolutionary younger genes were increasingly expressed towards the later timepoints of biofilm growth. In other words, we found that Bacillus ontogeny strongly recapitulates phylogeny. So far, these patterns have been considered the signature of embryo development in complex eukaryotes." said Domazet-Lošo.
The team searched for other embryogenesis characteristics in biofilms and found some, like an increase in the expression of genes involving multicellularity, or specific architectural characteristics that are linked to stages of an organism.
While more work will be needed before we can say that clusters of microbes are like multicellular animals, this work suggests that biofilms are far more complex than we appreciate.
"Our results point that a biofilm should be viewed as a multicellular individual, and not as a pile of individual cells. Like in animal embryogenesis, every developmental phase has its own peculiarities. Critical transition stages in biofilm growth could now be targeted via their stage-specific genes that we detected. This could be a game-changer in treating biofilm-related diseases, and in preventing industrial losses." concluded Domazet-Lošo.
Sources: AAAS/Eurekalert! via Rudjer Boskovic Institute, Molecular Biology and Evolution